Who Discovered Protons, Electrons and Neutrons? The discoveries of electrons, protons and neutrons were made by J. J. Thomson, Ernest Rutherford and James Chadwick respectively.parts. 1 Calculating Protons, Electrons, and Neutrons. 2 Calculating the Electrons with Ions Present. This article has been viewed 2,424,443 times. Finding the number of protons, neutrons, and electrons in a given element isn't as hard as it sounds.of Protons/ Electrons Number of Protons: 8 Atom Number of Electron: 8 Ion Number of Electron: 10 Atom Gain Electrons (-) Anion Atom Lose Electrons (-) Cation : − Full Electronic Configuration Diagram e.g. X -. Use pencil for Drawing -. Number of Protons and Neutrons in the...How much do protons, neutrons and electrons weigh? Protons and neutrons weigh 1 atomic mass unit each. An atomic mass unit (amu) is a tiny fraction of a gram. This is about 1.66 x 10-24 gram, a reaaally small number!Neutron Interferometer Facility (NIOF). Precision Imaging Facility. The databases ESTAR, PSTAR, and ASTAR calculate stopping-power and range tables for electrons, protons, or helium ions, according to methods described in ICRU Reports 37 and 49.
How to Find the Number of Protons, Neutrons, and Electrons
Protons and neutron are created by a process called Pair production. Pair production refers to the creation of an elementary particle and its antiparticle via Quantum Chromodynamics (QCD). Pair production can only occur if the photon has an energy exceeding the twice the rest mass(me) of an...Now, protons, and neutrons and electrons relative charge is listed right here. That would be the neutrons and the protons added together. This number does not come off a periodic table. If you look at a periodic table, there is something on that periodic table called atomic mass.These smaller particles - the protons, neutrons and electrons - all have different properties. Neutrons are large and heavy like protons, however neutrons have no electrical charge. Number of Protons and Neutrons - How to find the Atomic Number of an element Every element has a unique...In this video we'll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Aluminum (Al). From the...

Electrons, protons, neutrons
Draw a Aluminum Atom Protons: 13 Neutrons: 14 (27-13= 14) Electrons: 13. 6 Draw a Carbon Atom Protons: 6 Neutrons: 6 Electrons: 6. 7 Carbon "The building block of life" -carbon atoms are the basis of most molecules that make up living things Carbon forms covalent bonds with up to four other...Proton—positive; electron—negative; neutron—no charge. The charge on the proton and electron are exactly the same size but opposite. The same number of protons and electrons exactly cancel one another in a neutral atom. Note: The picture shows a simple model of the carbon atom.How many protons, electrons and neutrons are in an atom of krypton, carbon, oxygen, neon, silver, gold, etc...? To find the number of protons, electrons and neutrons in an atom, just follow these easy stepsSome atoms of aluminum do not have 14 neutrons. These atoms are called isotopes. Al-26 is an aluminum isotope with a half-life of 730,000 years. Electrons are the negatively charged particles in an atom. A neutral atom has no net charge, so the protons charges and electron charges must...How many electrons does this aluminum have? budwilkins budwilkins. 13 electrons when "neutral" or as it is on periodic table.
NameAluminumAtomic Mass26.982 atomic mass unitsNumber of Protons13Number of Neutrons14Number of Electrons13
Keeping this in view, how many protons and neutrons are in aluminum 27?
A quick seems within the periodic table will display that aluminium has an atomic number equivalent to 13 . This means that any atom this is an isotope of aluminium could have 13 protons in its nucleus.
How many orbitals does aluminum have?
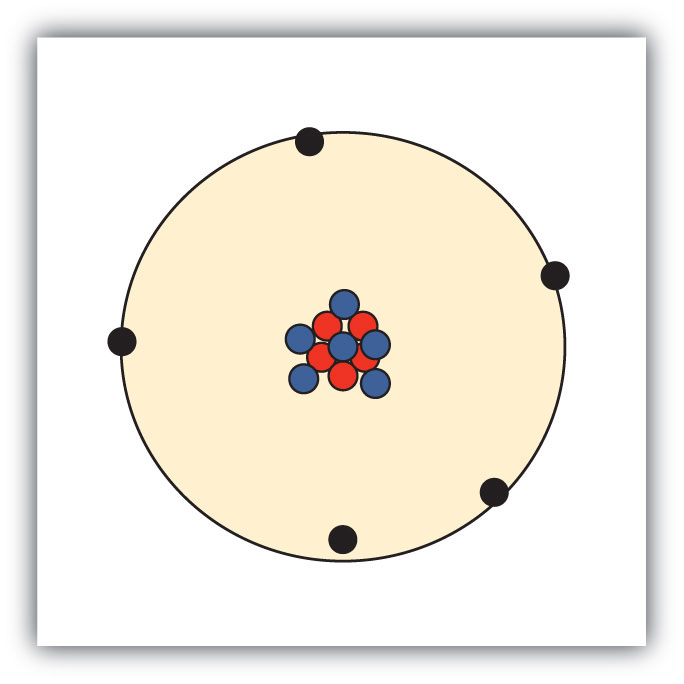
So for the element of ALUMINUM, you already know that the atomic number tells you the choice of electrons. That means there are 13 electrons in a aluminum atom. Looking at the image, you can see there are two electrons in shell one, eight in shell two, and three in shell 3.
How many electrons are in a +3 cation with an atomic choice of 13?
Here, mass number is 27 and there are 14 neutrons therefore protons=27-14=13. In a neutral atom, choice of protons=choice of electrons. Hence in this case the collection of electrons in the neutral atom will likely be 13. Now, because the atom carries +3 fee therefore it's going to have 13-3=10 electrons.
Who Discovered Protons, Electrons and Neutrons ...

Finding the Protons, Neutrons, Electrons, u0026 Mass ...

Aluminium - Protons - Neutrons - Electrons - Electron ...

Interactive notebook templates for atomic structure ...

Mckenzie

Matter and Atoms Review SpeedMatch Review Game

Aluminum Periodic Table Protons Neutrons And Electrons ...

Valence Electrons 3/26 - Devika's ISN

Unit 3 2 structure of the atom

Aluminum Periodic Table Protons Neutrons And Electrons ...

Atomic structure

Aluminum: Aluminum Neutrons
How are subatomic particles different from each other and ...
30 Bohr Diagram Of Fluorine - Wiring Diagram Database
Lithium atom | Plugon

How do solar cells work? | Bysolar, Inc. | Atom project ...

This is the Magnesium-24 isotope. It has 12 neutrons and ...

Bohr Model of Aluminum | Chemistry | Bohr model, Atom ...

PPT - Structure of the Atom PowerPoint Presentation, free ...

Aluminum Periodic Table Protons Neutrons And Electrons ...

rendu 3D de la structure de l'atome de chlore ...

0 comments:
Post a Comment